Haemoglobin, being the most direct link between the external situation and body requirements is one of the most interesting systems for studies of the inter-relationships between environmental conditions and molecular evolution. The Hb of species that live under extremely variable environmental conditions may have experienced a major evolutionary pressure to adapt and modify its functional properties. In the course of evolution, Hbs have developed a common molecular mechanism, based on the principle of ligand-linked conformational changes in a multi-subunit structure. Within the framework of this common mechanism, however, different Hbs have acquired special features to meet special needs (Guido, et al., 1991).
The bar-headed goose is a migratory bird which lives and hatches their young at Qinghai lake on the western plateau area of China but spends the winter in the northwestern part of India flying directly over the Himalayas with an altitude of about 9000m. The ambient atmosphere is very thin at the summit of the mountains, the P02 is around 50 mmHg which amounts to 30% of P02 at sea level (Swan, 1970). Physiological studies show that under conditions of progressive hypoxia, bar-headed geese show no significant polycythemia and increase of Hb concentration. Biochemical analysis shows that IPP levels are the same in erythrocytes of the bar-headed goose and its close lowland relatives, the greylag goose(Anser anser) and Canada goose (Branta canadensis) (Petschow et al., 1997). The Hbs from these birds have the same IPP binding contents. Purified bar-headed goose Hb shows only slightly higher oxygen affinity than that of greylag goose. This small intrinsic Hb oxygen affinity difference is amplified 10-fold in the presence of organic phosphate(IPP) and in 100mM chloride. This allows the bar- headed goose to uptake sufficient oxygen for flight from a very thin atmosphere, even at about 9000 metres above sea-level (Rollema & Bauer, 1979).
Former studies of bird high-altitude adaptation mechanisms are mainly by sequence analysis (Hielb, et al., 1986), abnormal Hbs (Perutz, 1983) and the study of mutant Hbs (Webber et al., 1993). All elucidated the importance of the mutation, which remove the hydrophobic contacts in alphal betal interchain to destablize the deoxy form Hb structure, therefore enhancing the intrinsic Hb oxygen affinity. In common with human Hb, bar-headed and greylag geese Hbs have 141 residues in the alpha subunit and 146 residues in the beta subunit. There are only four amino acid differenes in the major Hb types between bar headed goose and greylag goose, alpha 119Ser(bar headed) to Gly(greylag); alpha 63Ala to Val; alpha 119Ala to Pro; beta 125Asp to Glu, only one of which appears likely to affect oxygen affinity. The mutation of bar-headed goose, alpha 119Pro to Ala, has been introduced into humun Hb by genetic engineering and shown to cause an increase in oxygen affinity close to that found between blood from the two species of geese (Jessen et al., 1991). This being the first crystal structure of an avian Hb, we aim at looking for the features of steric structure at mutant positions between the bar-headed and greylag geese Hb, and whether additional changes may affect the control of oxygen binding.
The blood sample was drawn from the vein under the wing of a bar-headed goose and purified at 4! !. The oxyHb crystals were grown by the hanging drop method using 10% polyethylene glycol (PEG) 6000 in 50 mM pH 6.8 KH2P04-K2HP04 phosphate buffer. The crystals are tetragonal and in space group P4(2)2(1)2. The unit cell dimensions are a=b=81.59Angstrom, and c=107.28Angstrom. There is a dimer in the asymmetric unit(Hua et al., 1990).The metHb crystals were obtained under similar conditions as in the oxy form. The space group is P4(2)2(1)2, the cell parameters are a=b=81. 15Angstrom, c=107.20 Angstrom, alpha=beta=gamma90 degrees. There is also a dimer in the asymmetric unit(Lu et al., 1989). The deoxyHbs were prepared from the oxyHb with Na2S204 as reducing agent. The single crystals suitable for x-ray analysis have been grown from PEG 6000 at pH7.2 with protein 20mg/ml, also using the hanging drop method. DeoxyHb crystallizes in a P1 space group with unit cell dimensions a=70.90 Angstrom, b=95.40 Angstrom, c=58.72 Angstrom, alpha=72.8 degrees, beta=65.9degrees, gamma=82.0 degrees, the asymmetric unit has two molecules (Hua et al., 1996).
The x-ray diffraction data of the oxyHb were colleced at the Photon Factoryin Tsukuba, Japan using the Weissenberg camera of radius 429.7mm and a synchrotron radiation of 1.00 Angstrom (Sakabe, 1983). The intensity data were collected at 10degreesC using one crystal to 1.8 Angstrom. The data were processed with the Weiss program and an R merge value of 5.12%.The data of the oxyHb are 87% completeness to 2.0 Angstrom. The data of metHb were collected on an Area Detector X-200B and processed with program Xengen to 2.30 Angstrom resolution and an R merge value of 7.21%. The data of deoxyHb were colleted on the said Area Detector and processed to the 2.30 Angstrom, an R merge value of 7.00%. Recently, a data of the deoxyHb were collected at the KEK Photon Factory, Tsnkuba, with Macromolecular Weissenberg Camera (d=573mm), ring current 294mA, to about 1.90 Angstrom.
The molecular replacement method was used to obtain an initiai model for refinement. The coordinates of the human oxyHb (Shaanan, 1983) were taken as a model molecule for the bar- headed goose oxyHb, the coordinates of the horse metHb (Ladner, 1977) as a model molecule for the metHb. The coordinates of bar-headed goose oxyHb were taken as a model molecule for the deoxyHb. The structure refinements were carried out with simulated annealing by XLPOR (Brunger, 1992) and PROLSQ (Konnert, 1980), the manual adjustment of models to improve agreement with the calculated electron density map was done using TURBO-FRODO (Jones ,1982) on a SGI workstation, water molecules were added using the program ARP (Lamzin & Wilson,1993). The final model in oxy form has an R factor of 19.8%, calculated with all data between 10.0 and 2.0 Angstrom. The rms deviations in bond lengths and angles are 0.020 Angstrom and 0.055 Angstrom respectively(Zhang, 1996). The final model in met form has an R factor of 0.180 calculated between 10.0 and 2.3 Angstrom, the rms deviations of bond lengths and angles were 0.012 Angstrom and 2_25degrees respectively(unpublished data). The model in deoxy form has an R factor of 21.3%, calculated between l0_O and 2.3 Angstrom, the rms deviations in bond lengths and angles are 0_018 Angstrom and 3.10 degreesrespectively (unpublished data). Further work on deoxyHb will be continued. Final qualities of the bar-headed goose Hb molecule strutrues were checked by program PROCHECK (Laskowski, 1993).
Some findings resulting from our studies of Hb crystal structure are as follows. Since the sequences of bar-headed goose Hb are 69% identical with that of human Hb, the tertiary and quaternary structures of bar-headed gooseoxy Hb are similar to that of human Hb, having seven helices in the alpha chain and eight helices in the beta chain. The main differences in the structure occur at the subunit termini and surface residues. Superimposing the haem groups of the bird Hb on R state human Hb (excluding the haem side-group), it can be seen that the A helix and AB and GH corners of the alpha chain and the A and F helices and AB and GH corners of the beta chain are shifted. The beta chain shows larger differences than the alpha chain, particularly in the B helix. This is expected, since the beta chain N and C terminal residues are located at the Hb organic phosphate binding site, the allosteric regulators in bird and mammalian Hb are IPP and DPG respectively. Using haem Fe ion as the reference point, the distance from Fe of alphal chain to Fe of alpha2 chain is slightly larger than that of human oxyHb, but the distance from Fe of betal to Fe of beta2 is slightly shorter.
The alpha1 beta2 interface is a principal switch region controlling the transition between the high and low oxygen affinity forms of Hb. Amino acid changes in this region of the molecule are prime candidates for detailed analysis of a Hb with a profoundly altered oxygen affinity, however there are no mutations between bar-headed and greylag goose Hb at the alpha1 beta2 interface which appear to alter the oxygen affinity significantly. There are two amino acid substitutions between the human and goose protein, these are probably differences between mammalian and avian Hb in general and not adaptive to a specific environment.
The residues which are in contact with the haem group are conserved in vertebrate Hb. The residues which form the hydrophobic cage on the haem proximal and distal sides are exactaly the same in bar-headed goose and human Hbs. Compared with human Hb, the alpha subunit proximal histidine residues of bar-headed goose Hb adopt slightly more symmetrical positions on the haem axis. The sequences of the haem pockets between bar-headed goose and greylag goose Hb have no mutation, it is unlikely that any change in ligand binding is brought about by altered residues influencing the ligand directly.
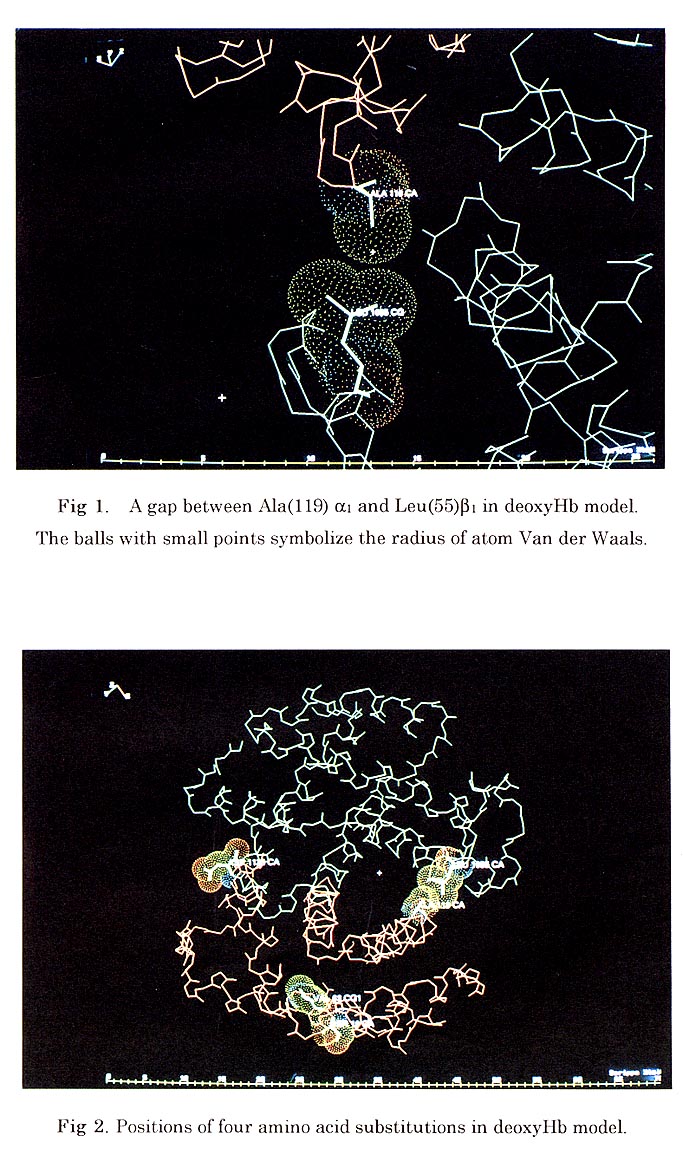
Generally the alphal betal contact region of bar-headed goose Hb involves the same residues as in human Hb, the helices B, G and H of the alpha subunit packing against the helices B, D, G and H in the beta subunit. When superimposed in the alpha1 beta1 interface "BGH core", the major structure difference is at the GH loop of the beta chain which is displaced relative to the other part of interface. Sequence comparison and protein engineering studies on human Hb have shown that the loss of the Pro(119) alpha to Leu(55) beta contact of the alphal betal interchain is critical for increased Hb oxygen affinity in the high altitude birds. In bar-headed goose Hb model, the Pro(119) alpha to Ala mutation appears to cause no significant change in protein structure. The main chain conformation of this area is very conserved despite the amino acid substitution. The Ala(119) adopts the Proline-like main chain conformation, which is "disallowed" in the Ramachandran plot. There is no interchain hydrophobic contact between Ala(119) alpha1 and Leu(55) betal in oxy and deoxy forms. The large gap between these two residues in deoxy form is slightly bigger than the one in oxy form (Fig. 1). The Asp (125) beta1 (Glu in greylag) is found near the alpha1 beta1 interface, but the direction of the side chain of the residue is going out and forms a hydrogen bond with water molecule, the other two amino acid substitutions also are found on the surface of molecule. (Fig. 2)

Vertebrate Hbs bind a variety of allosteric effectors in the deoxy state to reduce their oxygen affinity inside the red cell. The Hb of bird binds IPP around the 2-fold axis of Hb molecules. The IPP binding site in the Hb molecules is unaitered between bar-headed and greylag geese and may not therefore be involved in adaptation to high altitudes. Although the precise mode of binding of IPP can not be defined from our deoxyHb crystal structures at the present stage, but Val beta 1 , His beta 2, Lys beta 82, Arg beta 104, Arg beta 143, Arg beta 135, His beta 139, Lys beta 144 and His beta 146 form a pocket lined with positive charges between the beta chains. Four His residues (His beta 139 and His beta 146) and two Arg residues (Arg135) form the bottom of the pocket and Arg143 forms the wall (Fig. 3).
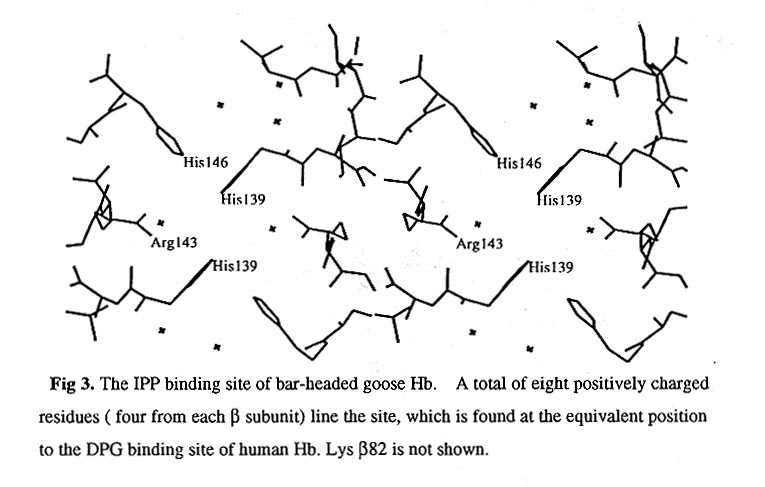
This positively charged region is undoubtedly the IPP binding site. Compared to human Hb, the IPP binding site of bar-headed goose Hb has more positively charged Arg and His residues, the strong positive charges appearing in this region are compatible with the strong binding of avian Hb to IPP. The significant conformational differences in this area between goose and human Hb may result from the different allosteric regulators they posses. It is of interest to note that the beta chain C terminal residue His beta 146 participates in IPP binding in goose Hb!! it makes the goose Hb IPP binding site become more strongly pH-dependent and the structural changes on IPP binding may more directly propagate to the alphal beta2 interface " switch region" and to the haem proximal residues. These may make the goose Hb more sensitive to the environmental changes.
References
Brunger, A. T.(1992). XPLOR version 3. 1, Yale University, CT, USA.
Guido, D. P., Saverio, G., Cond(, Maurizio, T., and Bruno G. (1991). TIBS 16-
December.Hiebl, I.,
Weber, R. E., Schneegans, D. & Braunitzer, G.(1989), Biol. Chem. Hoppe-Seyler, 370, 699-706.
Hua, Z. Q., Zhu, H., Wei, X. C., Lu, G. Y. & Gu, X. C. (1990). Acta Biophysica Sinica, Vol., 6, 2, 221-223.
Hua, Z. Q., Fang, y. L., Zhou, X. X., Xu, Q., Kuang B., Wei, X. C., Lu, G. Y.& & Gu, X. C. (1996). Acta Biophysica Sinica,Vol., 12, 3, 401-403.
Jones, T. A., (1982). In Computational Crystallography, pp. 3303-3317, Clarendon Press, Oxford.
Jones T.A., (1978). J. Appl. Crystallog. 11, 268-272.
Kimura, M. (1979). Sci. Arner. 241, 94-104.
Konnert, J. K. & Hendrickson, W. A. (1980). Acta Crystallog. sect. A, 36, 344--349.
Lamzin, V. S. & Wilson, K. S. (1980). Acta Crystallog. sect. D, 49, 129-147.
Laskowski, R. A., (1993). J. Appl. Crystallog. 26, 283-291.
Lu, G. Y., Mao, C. H., Wei, X. C., Hua, Z. Q., & Gu, X. C. (1989) Acta Biophysica Sinica Vol., 5 ,3, 260-262.
Pertz, M. F. (1983). Mol. Biol. Evol., 1, 1-28.
Rollema, H. S. & Bauer, C. (1979). J. Biol. Chem. 254,12038-12043.
Sakabe, N. ( 1983). J. Appl. Crystallog. 16, 542-547.
Shaanan, B. (1983). J. Mol. Biol. 171, 31-59.
Swan, L. W. (1979). Nat. Hist. 79, 68-75.
Weber, R. E., Hiel, I. & Braunitzer, G. (1988). Biol. Chem. Hoppe-Seyler, 369, 233-240.
Zhang, J., Hua, Z. Q., Jeremy, R. H., Lu, G. Y.,Zhang, R.J.& Gu, X. C.(1996). J. Mol. Biol., 255, 484-493.