Phycobiliproteins are the light-harvesting protein-pigment complexes in bacteria, algae and plants, absorbing light and transferring the energy to the photoactive reaction centers localized in the thylakoid membrane.
The major part of the light-harvesting system of the blue-green and red algae is formed by the rod-like phycobilisomes, which are composed of different phycobiliproteins arranged in a certain order in the phycobilisome: phycoerythrin is located at the tip of the rod, phycocyanin in the middle, and allophycocyanin forms the core that is attached to the stromol surfaces of the thylakoids . Energy transfer proceeds successively in the direction of phycoerythrin¨phycocyanin¨allophycocyanin¨chlorophyll a. with an overall efficiency of almost 100%[1,2,3].
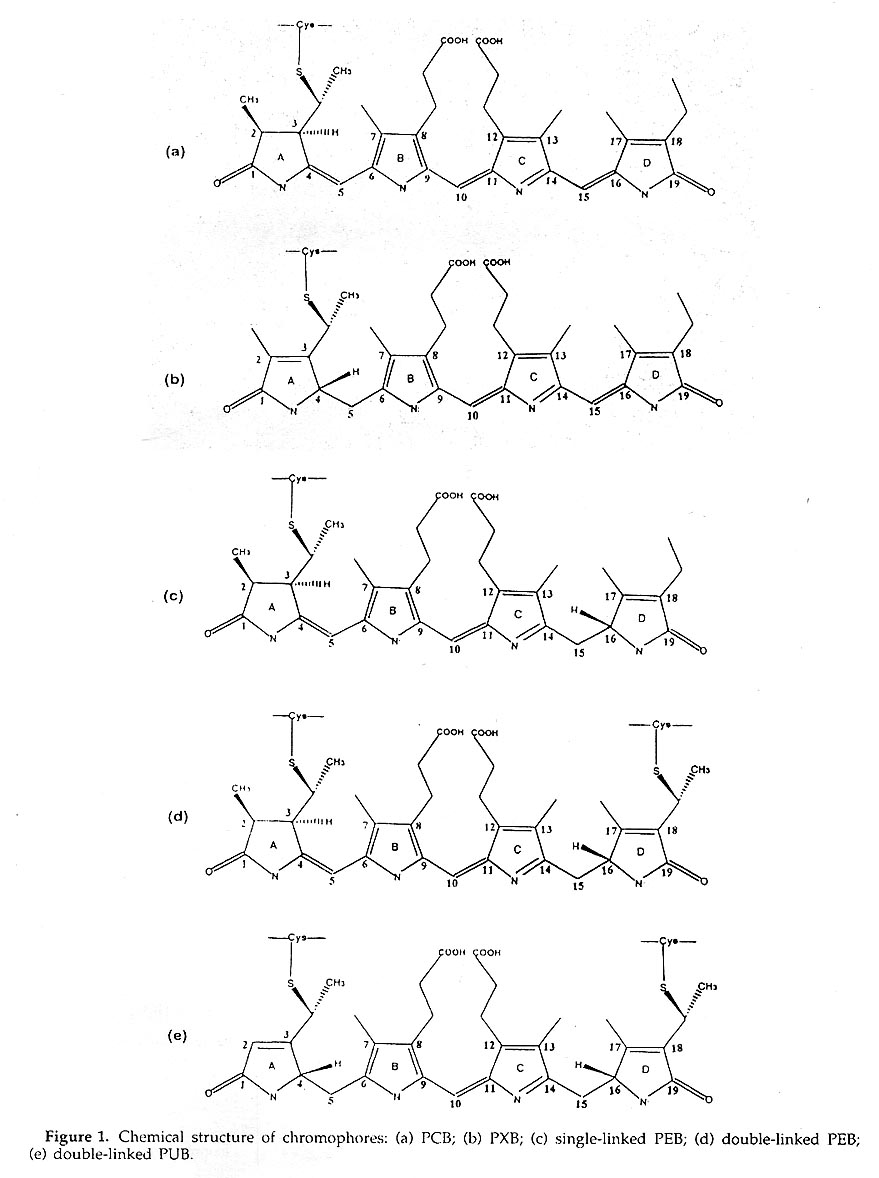
Phycobiliproteins all carry different numbers of chromophores, the structures of which are similar open-chain tetrapynoles, Linked via thioether bonds to cysteine residues and are classified by spectral differences as phycoerythrobilin(PEB), phycyanobilin(PCB), phycobiliviolin (PXB) and phycourobilin(PUB)(Figure1).
The crystals of R-phycoerythrin, R-phycocyanin and allophycocyanin ohained and all high- resolution data were collected by using Weissenberg camera KEK. The structure of all these phycobiliproteins have been or just determing in our group .

(1) R-PHYCOERYTHRlN
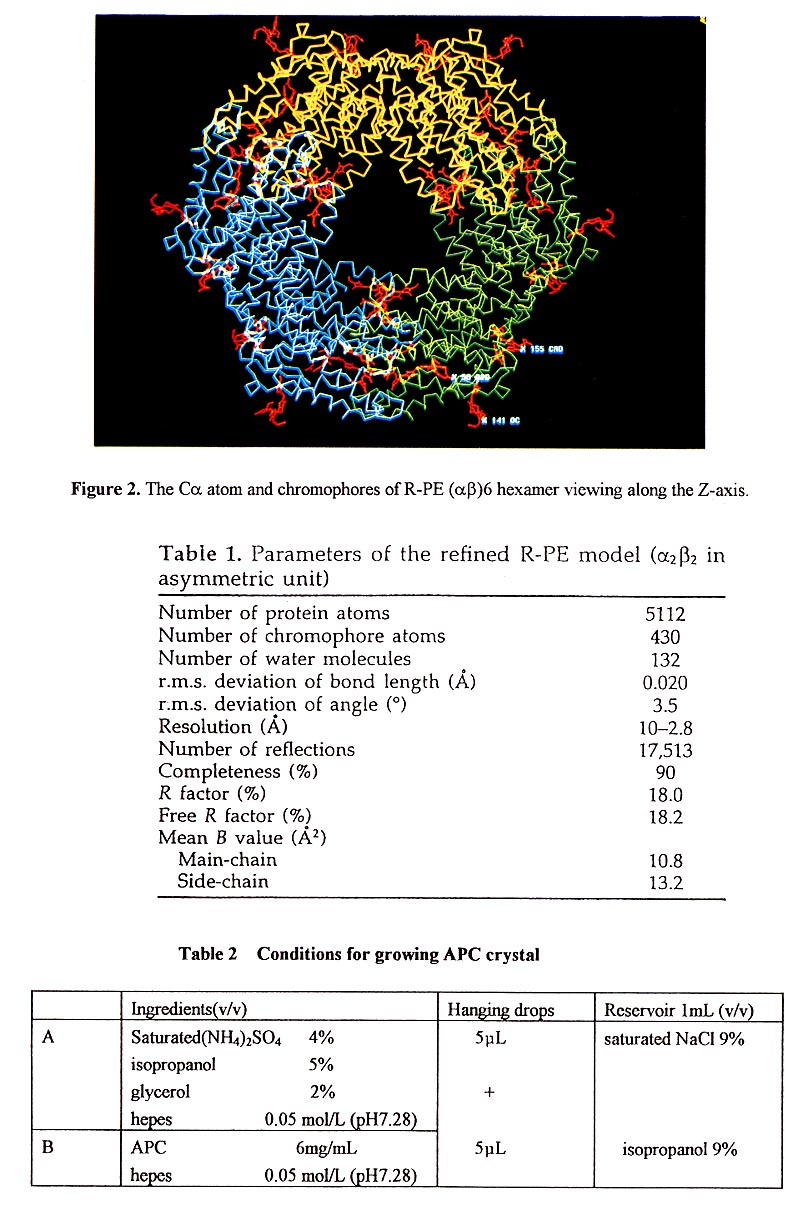
The structure of R-Phycoerythrin (R-PE) from Polysiphonia urceolata was determined at 2.8 ð resolution [4] The crystals belong to space group R3 with unit cell dimensions of a=b=189.8ð. c=60.1ð. The subunit composition of R-PE is (¿2À2)3Á. The three-dimensional structure of R-PE was solved by the multiple isomorphous replacement method. Reflection data of several kinds of derivatives were collected using Weissenberg camera in Photo Factory, KEK ,Japan. After several cycles of model building and refinement, the crystallographic R-factor of the final model is 18.0% with data from 8.0 to 2.8ð resolution. The final result is shown in Table 1.
Three (¿À) unit are arranged around a 3-fold symmetry axis to form an (¿À)3 trimmer, and two (¿À)3 trimmer are assembled face to face to build up an (¿À)6 hexamer, which is disc-shaped with a thickness of 60A and diameter of 110A. There is a channel in the center of the molecule with a diameter of 35ð(Figure 2). The ¿ and À subunits contain 164 and 177 residues, respectively, both have the same folding pattem and each subunit consists of nine helices and loops.
The four phycoerythrobilin(PEB) chromophores ¿84, ¿140a, À84 and À155 in an (¿À) unit are each covalently bound to a cysteine residue through ring A. A phycourobilin (PUB) chromophore is bound to a cysteine À50 by ring A and bound to cysteine À61 by ring D. The ring A and ring D of phycourobilin deviate from the conjugate plane formed by ring B and ring C and the four rings form a boat-shaped structure.
R-PE contains a 34K Da y subunit that is assumed to lie in the central channel of the molecular disc (¿2À2)3. The density in the central channel appears disordered and cannot be used for peptide tracing. The density of the 7 subunit in the channel appears disordered due to averaging by the 3-fold symmetry axis. The bindng of the Á subunit and (¿À) unit was assumed to be at a constant position, but orientations of the molecules ((¿2À2)3Á) in the crystal cell might differ by 120.
Most of the energy transfer mechanism was explained by Forster's dipole-dipole resonance and exciton interaction theory[5]. The approximate formula Ket=k2/Ñ0(R0/Á)6 is usually adopted for calculating the energy transfer rate where: Ket is the energy transfer rate; k2 is the orientation factor; R0=50A, is the Forster residue; Ñ0 is the fluorescence lifetime; and Y is the distance between two chromophores. The distance between 1¿84 and 6À84 is about 36A , which is suitble for the donor resonance and large for exciton interaction. But recently, the super-radiation of exciton interaction has been detected using a single crystal of R-PE, implying that exciton interaction plays an important role in R-PE energy transfer. It may be caused by the interactions between chromophores in the Á subunit and chromophores in the molecular disc (¿2À2)3, i.e. The position and orientation of chromophores in the Á subunit may bridge the distances between chromophores in the ¿ and À subunits.
The studies on the R-PE structure at high resolution (1.9A) is in progress. Detailed structural information will be reported in further studies.
(2)R-PHYCOCYANlN
Phycocymin can be classified into two groups as R-PC and C-PC by spectrum characteristic. C-PC is only carrying one kind of chromophore, PCB, but R-PC contains two kinds of chromophores, such as PEB and PCB. R-PC is the only phycobiliprotein containing PEB and PCB.
R-Phycocynain from polyslphonia urceolata has been isolated[6]. Single crystals of R-phycocyanin suitable for x-ray diffraction analysis have been grown in phosphate buffer and ammonium sulfate system by hanging drops vapor diffusion method. The crystals belong to tetragonal system with space group P41212 or P43212. The parameters of unit cell are: a=b=134.5ð, c=209ð, ¿=À=Á=90. There is one (¿À)3 molecule in an asymmetric unit. 3.3ð resolution data were collected in the National Laboratory of Biomacromolecules in China and recently 2.8ð data were collected in Photo Factory, KEK by means of synchrotron radiation source. The structure of R-PC was deternained by molecule-replacement and the refinement of module is just in progress.
(3)ALLOPHYCOCYANlN
Allophycccyanin fiom Porphyra yezoensis was purified. Single crystals were obtained by hanging drops vapor diffusion method at room temperature[7].The optimized crystallization conditions are given in Table 2. There are three (¿À)3 in an asymmetric unit. Because of the large molecular weight and the poor diffraction quality, crystals of APC with space group P4132 or P4332, a=b=c=286ð, ¿=À=Á=90 are useless in structure analysis. So we spent a lot of time to search for new crystallization condition and recently a new crystal form of APC, whose space group is R32 with cell dimension a=b=105.3ð, c=189.4ð, ¿=À=90, Á=120 was obtained. We are trying to improve the crystallization condition and collect high-resolution data. We hope the high resolution structure of three kinds of phyoobiliproteins R-PE, R-PC and APC could be completed soon and they would provide a structural basis for explaining the light harvesting and transferring mechanism.
Reference
Acknowledgement
We'd like to express our much thanks to Prof. Noriyoshi Sakabe and TARA(Tsukuba Advanced Research Alliance) for their kind support and help. We are very appreciated to the Photon Factory, KEK for providing the synchrotron radiation source and facility and to Dr. Kiwako Sakabe and Dr. Watanabe for giving us great help in arranging and providing the Weissenberg camera.