
1.TARA用実験ステーション
1.BL6Bの利用状況
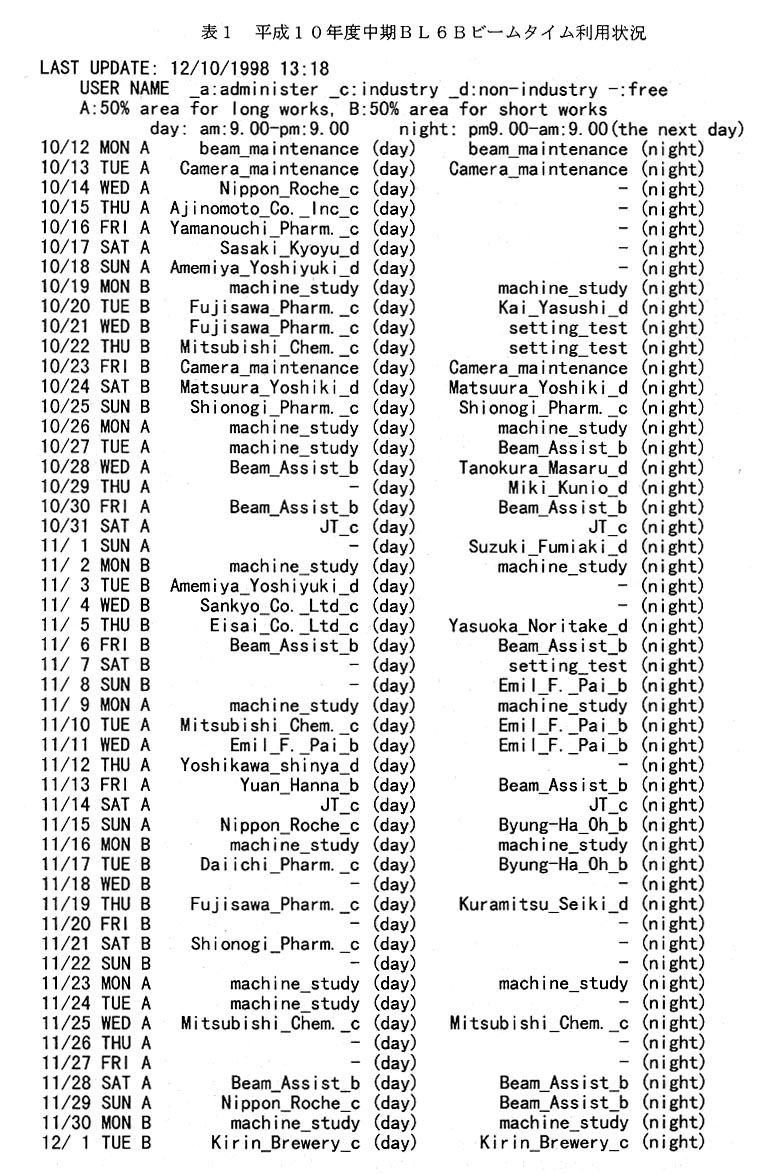
平成10年度中期のビームタイムは10月12日(月)から開始され12月23日(水) 迄である(表1)。前期と同様中期もCamera-maintenanceの名目で予備日を設け、1週間 前迄に急を要する要求がなければキヤンセルを行った。今期の特徴は此の予約表が頻繁に 変わった事である。即ちキヤンセルがあり別の利用者がをそれを見て埋め効率よく利用さ れた。恐らく結晶が思うように出来なかったのであろう。これは利用者が予約システムに 償れ、予約システムが本来の機能を発揮し始めたのであろう。
平成10年度後期のビームタイムは1月29日(金)~2月25日(木)である。その 予約状況を表2に掲げる。後期は一ケ月弱という短期間のためか既に昼夜共にほとんど空 きが無い状態である。急な要求に対しては出来るだけCamefa-maintenanceの日を対応させ る。もう一つ特徴的なこととして、大学関係者による登録が減ってきている。これは大学 関係者の要求が減ったわけではなく、年間2駒即ち24時間という制限のため、既に殆ど の大学関係者は制限時間のため予約出来なく成っていると思われる。これについては BL6Cの運用を含め今後検討を行う予定である。
大型IP読取装置の利用者が急増した。利用者から大型IP読取機のトラブルについて の苦情をあまり聞かなくなったので、これは大型IP読取機が安定しトラブルが減った為 と思っていた。しかし、積極的に利用者やビームラインアシスタントに尋ねるとIPの搬 出等のトラブルは減ってはいるが未だ起こっているとのことである。それにも係わらず大 型IPの利用が増したのは、利用者がトラブルの対処方法を心得て夜間も恐れることなく 利用出来るように成った為であろう。
2.ビームラインアシスタント
今期ビームラインアシスタントの方々の名輝を掲載し感謝の意を表します。


Ⅱ.TARA第2実験ステーションBL6C
BL6Cビームライン及びハッチに関する建設は計画通り実行された。日本学術振興会 未来開拓推進事業の産学連携研究費で開発中の全自動データ収集システムの内今年度迄の予算で開発した部分を予定より早く後期のビームタイムが始まる前にBL6Cハッチ内に 設置する予定で頑張っている。
Ⅲ.コンピュータ関係
共同利用が開始された10月12目より11月30日までの利用状況を報告する。
1.ネットワークとデータサーバの状況
10月のビームタイムが始まる前、サーバのメモリーを今までの128MBに512MBを 追加増設した。これは今期から2台のDEC500auが増設されサーバに対する負荷の増加に 対処するためと、メモリーの増設が今後のデータ転送量の増加に対処するのに最も有効だと考えた為であった。
9月にサーバのディスク1台が故障していることが分かった。実際にはこのディスクが 故障した時点で、正常な補助ディスクと自動的に入れ替わったので直接的な影響はなかったが、RAlDシステムを採用していなかったら少なくとも半目間はサーバがストップしデ ータ測定に影響が出ていたものと推測された。この1年の間にはディスクの故障でサーバ が停止する事故はまだ起きていない。
10月の初めから始まった今期はユーザのデータ量が急増し、データ保存期間を3日間 に短縮した時期が10月22日~10月30日、11月17日~11月21日と2回あっ た。現在学振未来開拓により9GBディスク6台を発注し、来年1月のビームの出ていな い時期を利用し、サーバに増設する事が予定されている。従って、次期のビームタイムでは状態の改善が期待できる。またユーザに不要なファイルの消去をお願いした効果もあ り、現在は保存期間を7日間に戻して運用をしている。尚、変更時にはe-mail によりTARAユーザーを含む蛋白用ビームラインの全ユーザーに連絡を行っている。
TARA第2ステーションBL6Cに設置する自動データ収集システムに関する研究補 助を今後官本康弘氏に行ってもらう。その為には官本康弘氏に本システムに関する知識及ぴ技術を十分収得してもらう必要がある。そこで、平成10年7月8日より平成11年1 月20日(本体搬入予定日)迄、本装置を製作しているマックサイエンス加賀工場に派遺 する事にした。その間TARAハウス内のネットワークに関する業務は学振未来開拓のポ スドクである伊藤和輝氏にお願いした。
2.実験ステーション用ワークステーションの機種更新
BL6A、BL18Bに設置してあったDEC3600の2台をDEC500auの2台に置き換え、BL6B に設置されていたDEC3700をTARAプレハブの2階に設置してあったDEC433au 1台で 置き換えた。これらは全てDECAlphaServer 4000とNFS/NlSで接続した。この変更に伴 ってBL6Bに移設したDEC433au、TARAプレハブ計算機室内のINDlGO2(R10000)の転 送速度が非常に遅いとの苦情がユーザから寄せられ、その原因を多角的に検討したが解明できなかった。そこで応急処置としてINDlGO2(R10000)をBL18B側のネットワークに繋 ぎ替えることにより以前のスピードに戻すことができた。またDEC433auに関しては元の ホスト名(tarade4)に戻すことにより64MBのデータを7~9秒で転送できるようになっ た。根本的な解決は後日行う予定である。
3.その他
バツクアツプのDLTテープがローダから戻らなくなる事故が10月23日に起きて、 次の日に2日分をバックアップしたが、保存期間内なので問題はなかったと思われる。前 回に間題になった回線数の不足については、不要なWeb接続をやめるようにお願いした ためか問題としては上がってこなかった。今後ともサーバ上の不要ファイルの消去、および不要なインターネットへの接続をしないようご協カをお願いしたい。
Ⅳ.各種委員会報告
1.第5回運営委員会
平成10年9月9日、TARA坂部プロジェクト第3回総会がTARAハウス2階・ト レーニングホールで開催された。それに先立ち同ホールで第5回運営委員会が開催された。まずプロジェクト代表祥雲弘文教授よりこれまでの研究プロジェクトは3年の期間を終 了したが、本年4月より本プロジェクトは筑波大学先端学際領域研究センター(TARA) のリエゾーン推進特別プロジェクトとして新たに発足した事、及びその経過について詳しい報告がなされた。その後本誌「活動報告」及びそれを補う形で一般活動報告がなされた。続いて議事がなされた。まず坂部運営委員長から平成9年度収支報告がなされた。大き な変更点として、TARA用第2ステーションBL6Cの建設許可が平成10年2月になった ためその建設費は平成10年度に持ち越されたことである。その他TARAハウス用什器 など主な備品費項目10点、TARAハウス内弱電工事等3点、計算機関連項目10点な どが示され説明がなされた。その後山崎憲一委員より監査報告がなされた。審議後平成9 年度収支報告が認められた。平成10年度予算案が坂部知平運営委員長より提案された、 平成9年度より繰り越されたTARA用第2ステーション建設費65,000千円以外は例 年に準じており、審議の結果原案通り承認された。
山崎憲一会計監査役が川上善之氏と交代する事が認められた。交代に当たり川上善之氏 から会計監査の役割についての質問があり坂部知平運営委員長より次の説明がなされた。本プロジェクトの寄附金は国際科学振興財団(FAlS)が管理しており公認会計士が 会計監査を行っている。又筑波大学にある委任経理金は大学が管理している。従って今回質問のあった会計監査役には法的な責任は一切ない。ミスを無くするため坂部知平もFA ISに支払いを依頼した伝票のコピー及びパソコン上に収支簿を付け、FAISから提出 された収支簿をチェックしている。これらの資科を坂部プロジェクトメンバー全員に配布する代わりに会計監査役と企業代表に収支簿を送りチェックをお願いしている。必要に応じ伝票のコピーも見ていただいている。
栗原宏之編集委員長が石川弘紀氏と交代することが認められた。畠忠運営委員会議長よ り平成10年中期以降のビームタイム配分方法について意見が求められ、討議の結果当分 の間現状維持と決定された。
運営委員会議案を表3に示した。

2.新旧合同編集委員会
第10回編集委員会が平成10年11月11日(水)18時よりTARAハウスにて新 旧合同で開催された。
構造生物Vo1.4,No.3の原稿の最終チェックならびに印刷等のスケジュールの確認が行わ れた。続いて次号(Vo1.5,No.l)の内容について検討が行われ、執筆依頼者及ぴ各委員の 役割分担が決定された。
3.行事委員会
三菱化学㈱の杉浦郁子氏が第5回運営委員会で、新たに行事委員に選任された。平成 10年10月16日(金)19時より、東京・二子玉川にて拡大行事委員会が開催された。
出席者は畠忠行事委員長(三共㈱)、杉浦郁子新行事委員(三菱化学㈱)、ゲストとして 杉尾成俊(三菱化学㈱)及ぴ特別ゲストとして川口洋子(三共㈱)の4名である。
本年7月7日に開催されたTARA坂部プロジェクトジョイントセミナー「蛋白の発現 から結晶化まで」の反省を行った後、今後の方針を決定した。
Ⅵ.業績紹介
TARAプロジェクトとして行った研究であることが明確である論文のみを掲載する。 尚、本プロジェクトのメンバー名と所属を各論文の文頭に掲げた。
1.祥雲弘文(筑波大)
Site-Directed Mutagenesis of the Conserved Threonine (Thr243) of the Distal Helix of Fungal Cytochrome P450nor Biochemistry 37. 25. 8839-8847 (1998)
Noriaki Okamoto1, Yoshio Imai1. Hirofiuni Shoun2, and Yoshitsugu Shiro3 1Departuent of Veterinary Science, Osaka Prefecture University, Sakai, Osaka 599-8531. Japan.
2Institute of Applied Biochemistry and Sakabe Project at the Center for Tsukuba Advanced Research Alliance (TARA), University of Tsukuba. Tsukuba, lbaraki 30-58572. Japan.
3The Institute of Physical and Chemical Research (RIKEN), RIKEN Harima Institute, Mikazki-cho, Sayo. Hyogo 679-5143, Japan
Summary
Cyiochrome P450nor (P450nor) is a heme enzyme which catalyzes NO reduction in denitrifying fungi. Threonine 243 (Thr243) of P450nor, which corresponds to the conserved threonine of monooxygenase cytochrome P450s, was replaced by I 8 different amino acids via site-directed mutagenesis. The mutation did not seriously affect the optical absorption and the CD spectral properties of the enzyme in several oxidation, Iigation, or spin states or the association rate constant for association of NO with the ferric iron, suggesting subtle and local structural changes in the heme environment on Thr243 mutation. However, the NO reduction activity was dramatically altered by Thr243 mutation, depending on the properties of the replaced amino acids. The catalyiic activity, as measured by N,O formation and NADH consumption, was considerably retained on substitution of Asn, Ser, and Gly for Thr243, while it was profoundly decreased or lost on substitution with other amino acids. Kinetic analysis of the reaction of the enzymes with NO and NADH indicated that the decrease in the enzymatic activity upon Thr243 mutation mainly results from a decrease in the rate of reduction of the ferric-NO complex with NADH. On the basis of these enzymatic, kinetic, and spectroscopic results, as well as on the basis of the crystal data for native P450nor [Park, S.-Y., et al. (1997) Nat. Struct. Biol. 4, 827-832], the role of the conserved threonine at the 243 position in the NO reduction reaction by P450nor is discussed. We also discuss structural similarities or differences in the vicinity of the conserved threonine between P450nor and other monooxygenase P450s.
TARAに関する表現
所属の欄に記載
2.祥雲弘文(筑波大)
Denitrification by Actinomycetes and Purification of Dissimilatory Nitrite Reductase and Azurin from Streptomyces thioluteus Journal of Bacteriology 180, 17, 3413-4415 (1998)
Hirofumi Shoun1,2, ,Mitsuyoshi Kano1. Ikuko Baba1 , Naoki Takaya1, and Masaru Matsuo1
Institute of Applied Biochemistry1 and Center for Tsukuba Advanced Research Alliance (TARA),2
University of Tsukuba. Tsukuba. lbaraki 305-8572, Japan.
Summary
Many actinomycete strains are able to convert nitrate or nitrite to nitrous oxide (N2O). As a representative of actinomycete denitrification systems, the system of Streptomyces thioluteus was investigated in detail. S. thioluteus attained distinct cell growth upon anaerobic incubation with nitrate or nitrite with concomitant and stoichiometric conversion of nitrate or nitrite to N2O. suggesting that the denitrification acts as anaerobic respiration. Furthermore, a copper-containing, dissimilatory nitrite reductase (CuNir) and its physiological electron donor, azurin, were isolated. This is the first report to show that denitrification generally occurs among actmomycetes.
TARAに関する表現
所属の欄に記載
Aknowledgements; This work was supported by ------------- and the TARA Sakabe Project of University of Tsukuba ---------.
3. 岡村直道(筑波大)
Post-Meiotic Expression of the Mouse Dihydropyrimidinase-Related Protein 3 (DRP-3) Gene During Spermiogenesis
Molecular Reproduction and Development 51. 105-111 (1998)
Yoichi Kato1 .Naoki Hamajima2 .Hiroshi Inagaki3 .Naomichi Okamura4 .Takehiko Koji5, Makoto Sasaki1 and Masaru Nonaka6
1Department of Biochemistry, Nagoya City University Medical School, Mizuho-ku. Nagoya. Japan
2Department of Pediatrics. Nagoya City University Medical School. Mizuho-ku. Nagoya. Japan
3/Departnlent of Pathology, Nagoya City University Medical School. Mizuho-Ku. Nagoya. Japan
4Institute of Basic Medical Sciences and Center for Tsukuba Advanced Research Alliance, University of Tsukuba, Tsukuba, Ibaraki. Japan
5Department of Histology and Cell Biology. Nagasaki University School of Medicine. Nagasaki, Japan
6Department of Biological Sciences, Graduate School of Science. University of Tokyo. Hongo, Tokyo, Japan
Summary
The dihydropyrimidinase-related protein (DRP) family, originally identified in hunlans by their homology to dihydropyrimidinase, contains at least four members. Genes of this family, and their counterparts in other manunals and chickens. are expressed mainly in fetal and neonatal brain, suggesting that the encoded proteins have a physiological role in the development of the central nervous system. In addition. the DRP-3 gene is expressed in testis as a shorter mRNA than the brain form. As a first step in understanding the extra-neuronal function of DRP-3. the structure and expression of testis DRP-3 were examined. Testis DRP-3 CDNA showed the same sequence as brain DRP-3 CDNA. except for the 5'-terminal end, which encodes a 5'-untranslated region and the I I N-terminal amino acid residues. indicating that the two forms of DRP-3 mRNA were transcribed from a single copy gene. Northem blotting analysis detected DRP-3 mRNA in 30-. 40- and 70-day-old. but not in lO- and 20-day-old testes. In situ hybridization analysis indicated that the expression of DRP-3 in testis is restricted to post-meiotic round spermatids. This is the first report of the expression of DRP genes in extra-neuronal cells.
Key Words; DRP-3; testis; in situ hybridization
TARAに関する表現
所属の欄に記載
4.馬場 忠(筑波大)
p-Aminobenzamidine-sensitive acrosomal protease(s) other than acrosin serve the sperm penetration of the egg zona pellucida in mouse Zygote 6 . 311-319(1998)
Kazuo Yamagata. Keitaro Murayama, Nobuhisa Kohno. Shin-ichi Kashiwabara and Tadashi Baba
Institute of Applied Biochemistry (IAB) and Tsnkuba Advanced Research Alliance (TAIRA).
University of Tsukuba. and the National Institute for Advanced Interdisciplinary Research (NAIR), Tsukuba Science City, Ibaraki, Japan
Summary
It has been reported that a significant delay in protein dispersal from the acrosomal matrix is observed in wild-type sperm by adding p-aminobenzamidine, a trypsin/acrosin inhibitor, to the incubation medium. The pattern of this delayed release was similar to that of the acrosin-deficient mutant mouse sperm (Yamagata et al., J. Biol. Chem., 273, 10470-4, 1988). In the present study, no further delay in protein dispersal was found when the acrosin-deficient sperm were treated with p-aminobenzamidine, indicating that among the p-aminobenzamidine-sensitive protease only acrosin may function to accelerate this process. Although the acrosin-deficient sperm penetrated the zona pellucida (Baba et al., J. Biol. Chem., 269, 31845-9, 1994), the addition of p-aminobenzanridine to the fertilisation mediunl caused a significant inhibition of fertilisation in vitro. This indicates that there is a p-aminobenzamidine-sensitive protease other than acrosin participating in the zona penetration step. Indeed, we demonstrated that a non-acrosin protease with a size of 42 kDa was present in the supematant of the acrosome-reacted sperm suspension. The enzyme was inhibited by p-amimobenzamidine, diisopropyl fluorophosphate and Nα -tosyl-L-Iysine chloromethyl ketone, and was apparently activated by acrosin.
Keywords: Acrosin, Egg, Mouse, Sperm, Zona pellucida
TARAに関する表現
所属の欄に記載及び
Acknowledgements; This study was partly supported by ------------ and by TARA Sakabe/Shoun-Project --------.
5.馬場忠(筑波大)
Acrosin Accelerates the Dispersal of Sperm Acrosomal Proteins during Acrosome Reaction*
The Journal of Biological Chemistry 273., 17, 10470-10474 (1998)
Kazuo Yamagata, Keitaro Murayama. Masaru Okabe1, Kiyotaka Toshimori2, Tomoko Nakanishi1, Shin-ichi Kashiwabara, and Tadashi Baba
The Institute of Applied Biochemistry and Tsukuba Adcanced Research Alliance. University of Tsukuba, and the National Institute for Advanced Interdiscrplinary Research, Tsukuba Science City. Ibaraki 305-8572,
1the Research Institute for Microbial Diseases, Osaka Unirrersity, Yamadaoka 3-1, Suita, Osaka 565-0871
2the Department of Anatomy, Miyazaki Medical College. Miyazaki 889-1692, Japan
Summary
Using homologous recombination, we have previously produced male mice carrying a disruptive mutation (Acr-/-) in the acrosin gene. Although Acr-/- mouse sperm lacking the acrosin protease activity still penetrated the zona pellucida and fertilized the egg, the mutant sperm exhibited a delay in penetration of the zona pellucida solely at the early stages after insemination. To further elucidate the role of acrosin in fertilization, we have examined the involvement of acrosin in the acrosome reaction of sperm using the Acr-/- mutant mice. When the ability of sperm to adhere (attach) and bind to the zona pellucida of cumulus-free eggs was assessed in vitro, no significant difference was observed among Acr+/+ ACT+/-, and Acr-/- mouse sperm. Immunocyiochemical analysis demonstrated that the release of several acrosomal proteins from the acrosome of Acr-/-mouse sperm was significantly delayed during the calcium ionophore- and solubilized zona pellucidainduced acrosome reaction, despite normal membrane vesiculation. These data indicate that the delayed sperm penetration of the zona pellucida in the Acr-/- mouse results from the altered rate of protein dispersal from the acrosome and provide the first evidence that the major role of acrosin is to accelerate the dispersal of acrosomal components during acrosome reaction.
TARAに関する表現
所属の欄に記載及び
Footnote; * This work was partly supported by ------and by the Tsukuba Advanced Research Alliance Sakabe/Shoun Project (to T.B.).
6. 馬場忠(筑波大)
Two Novel Testicular Serine Proteases, TESP I and TESP2, Are Present in the Mouse Sperm Acrosome
Biochemical and Biophysical Research Communications 245. 3, 658-665 (1998)
Nobuhisa Kohno*. Kazuo Yamagata*, Shigehiro Yamada* , Shin-ichi Kashiwabara*, Yasuhiro Sakai** . and Tadashi Baba*
*Institute of Applied Biochemistry and Tsukuba Advanced Research Alliance (TARA), University of Tsukuba and the National Institute for Advanced Interdisciplinary Research (NAIR), Tsnkuba Science City, Ibaraki 305-8572. Japan
**Department of Anatomy. School of Medicine. Kitasato University. Sagamihara. Kanagawa 228-8555, Japan
Summary
To identify a novel candidate(s) for acrosomal proteins that act on the sperm/egg interaction, a DNA fragment was PCR-amplified from a cDNA Iibrary of acrosin-deficient mouse testis and then used as a probe to screen a mouse testis cDNA Iibrary. Complementary DNA clones encoding each of two similar but different serine proteases, TRSPI and TRSP2, have been identified. The nucleotide sequences of these clones indicate that mouse TRSPI and TRSP2 are initially synthesized as preproproteins of 367 and 366 amino acids, respectively. Comparison of the two TRSP sequences with those of typical serine proteases suggests that each TRSP zymogen is probably converted into a two-chain mature enzyme consisting of light and heavy chains covalently linked by a single pre-existing disulfide bond. The conversion may be accomplished by another protease(s) with a trypsin-like cleavage specificity, since it is unlikely that the mature TRSPI and TESP2 are capable of splitting the Lys-lle bond between the light and heavy chains. Northern blot analysis of total cellular RNA demonstrates that the TESPI and TESP2 genes are expressed only in the testis, and the transcripts are abundantly present in the haploid round spermatids. Moreover, immunocyiochemical analysis of mouse cauda epididymal sperm using afflnity-purified antibodies reveals that these two TRSPS are both localized in the sperm acrosome and are released during the acrosome reaction induced by calcium ionophore A23187. These findings provide additional clues
TARAに関する表現
所属の欄に記載及び
Acknowledgements; This study was partly supported by ------------and by TARA Sakabe/Shoun-Project -----------.
7. 田中勲、中川敦史(北大)
Escherichia coli positive regulator OmpR has a large loop structure at the putative RNA polymerase interaction site
Hidemasa Kondo1 ,Atsushi Nakagawa1. Jun Nishihira2, Yoshifumi Nishimura3, Takeshi Mizuno4 and Isao Tanaka1
1Division of Biological Science. Graduate School of Science, Hokkaido University, Sapporo 060, Japan
2Central Research Institute, School of Medicine. Hokkaido University, Sapporo 060, Japan
3Graduate School of Integrated Science. Yokohama City University, Yokohama 236. Japan
4Laboratory of Microbiology, School of Agriculture, Nagoya University. Nagoya 464. Japan.
The C-terminal DNA-binding domain of OmpR, a positive regulator involved in osmoregnlation expression of the ompF and ompC genes in Escherichia coli has a helix-turn-helix variant motif. The 'turn' region, consisting of I I residues, forms an RNA polymerase contact site.
TARAに関する表現
Acknowledgements; A.N. and I.T. are members of the TARA project of Tsukuba University, Japan.
8. 田中勲、中川敦史(北大)
Crystallization and Preliminary X-Ray CrystallographiG Study of the Ribosomal Protein S7 from Bacillus stearofaermophilus
Journal of Structural Biology 120, I12-114 (1997)
Nao Harada. Kazunari Sano, and Makoto Kimura Laboratory of Biochemistry. Faculty of Agriculture, Kyushu University. Fukuoka 812-81. Japan
Harumi Hosaka, Atushi Nakagawa, and Isao Tanaka
Division of Biological Sciences, Graduate School of Science. Hokkaido University. Sapporo 060, Japan
Summary
Overproduction and crystallization of Bacillus stearothermophilus ribosomal protein S7 (BstS7). a primary I 6s rRNA binding protein and also a translational repressor protein. have been performed to analyze its three-dimensional structure by X-ray crystallography. Ribosomal protein BstS7 was expressed in the cyioplasmic fraction of the E. coli cells and purified to homogeneity. This recombinant BstS7 was used to produce crystals with P2, symmetry that diffracted to 2 .5 A resolution which are suitable for high-resolution X-ray crystallographic analysis.
TARAに関する表現
Acknowledgements; A.N. and I.T are members of the TARA project of the University of Tsukuba.
9. 田中勲、中川敦史(北大)
Ribosomal protein S7: a new RNA-binding motif with structural similarities to a DNA architectural factor
Structure 5. 9. I 199-1208 (1997)
Harumi Hosaka1, Atsushi Nakagawa1, Isao Tanaka1. Nao Harada2, Kazunari Sano2, Makoto Kimura2, Min Yao3,4 and Soichi Wakatsuki3
1Division of Biological Sciences, Graduate School of Science. Hokkaido University, Sapporo 060, Japan.
2Laboratory of Biochemistry. Faculty of Agriculture, Kyushu University. Fukuoka 812. Japan.
3European Synchrotron Radiation Facility, BP 220, F-38043. Grenoble Cedex. France
4MAC Science, 1-5-1 Shinyokohama. Kohokuku, Yokohama, Kanagawa 222, Japan.
Summary
Background: The ribosome is a ribonucleoprotein complex which performs the crucial function
of protein biosynthesis. Its role is to decode mRNAS Within the cell and to synthesize the
corresponding proteins. Ribosomal protein S7 is located at the head of the small (30s) subunit of
the ribosome and faces into the decoding centre. S7 is one of the primary 16s rRNA-binding
proteins responsible for initiating the assembly of the head of the 30s subunit. In addition, S7 has
been shown to be the major protein component to cross-link with tRNA molecules bound at both
the aminoacyl-tRNA (A) and peptidyl-tRNA (P) sites of the ribosome. The ribosomal protein S7
clearly plays an important role in ribosome function. It was hoped that an atomic-resolution
structure of this protein would aid our understanding of ribosomal mechanisms.
Results: The structure of ribosomal protein S7 from Bacillus stearofuermophilus has been solved
at 2.5 A resolution using multiwavelength anomalous diffraction and selenomethionyl-substituted
proteins. The molecule consists of a helical hydrophobic core domain and a β-ribbon arm
extending from the hydrophobic core. The helical core domain is composed of a pair of entangled
helix-turn-helix motifs; the fold of the core is similar to that of a DNA architectural factor. Highly
conserved basic and aromatic residues are clustered on one face of the S7 molecule and create a
16S rRNA contact surface.
Conclusions: The molecular structure of S7, together with the results of previous cross-linking
experiments. suggest how this ribosomal protein binds to the 3 ' major domain of I 6S rRNA and
mediates the folding of I 6S rRNA to create the ribosome decoding centre.
TARAに関する表現
Acknowledgements ; A.N. and I.T.are members of the TAIRA project of Tsukuba University, Japan
10. 田中勲、中川敦史(北大)
Crystallization and Preliminary X-Ray Analysis of Human D-Dopachrome Tautomerase
Journal of Structural Biology 120, 105-108 (1997)
Hiroshi Sugimoto. Masae Taniguchi. Atsushi Nakagawa, and Isao Tanaka
Division of Biological Sciences, Graduate School of Science. Hokkaido University, Sapporo 060, Japan
and Masaki Suzuki and Jun Nishihira
Central Research Institutes. School of Medicine. Hokkaido University, Sapporo 060, Japan
Summary
D-Dopachrome tautomerase catalyzes the conversion of
dopachrome to 5,6-dihydroxyindole. This protein
has amino acid sequence homology with that
of macrophage migration inhibitory factor
(MIF), suggesting a pathophysiological role
of this protein in inflammatory and immunological
events. We previously determined the tertiary
structure of MIF and revealed the functional
and evolutional relationships of this protein
to isomerase. However, the reaction mechanism
of both proteins associated with the inflammatory
response. immune system. or tautomerase activities
in vitro have not yet been clarified. The
tertiary structure of *-dopachrome tautomerase
would provide insight into the molecular
function and the mechanism of these proteins.
In this study, we crystallized human D-dopachrome
tautomerase by a hanging-drop vapor diffusion
method. The crystals belong to the trigonal
space group P3, with unit cell dimensions
a = b = 84.2 Å and c = 41.0 Å. They contain
three (or two) monomers in the asymmetric
unit, corresponding to a VM value of 2.21 (or 3.32) A3 Da-1. The best crystals diffract X-ray to 1.6
Å resolution using a synchrotron radiation
source. Crystallization of the selenomethionyl
derivative of the protein for applying the
multiwavelength anomalous diffraction method
was also successful.
TARAに関する表現
Acknowledgements; A.N. and I.T.are members of the TARA project of Tsukuba University, Japan
11. 田中勲、中川敦史(北大)
Crystallization and Preliminary X-Ray Studies of Two Serine Proteinase Inhibitors. BGIA and BGIT, fiom the Seeds of Bitter Gourd
Journal of Structural Biology 120. 204-206 (1997)
Hidemasa Kondo. Jin Nakanose, Atsushi Nakagawa. and Isao Tanaka
Division of Biological Sciences, Graduate School of Science. Hokkaido University. Sapporo 060 Japan
and Makoto Kimura and Gunki Funatsu
Laboratory of Protein Chemistry and Engineering, Faculty of Agriculture. Kyushu University, Fukuoka 812. Japan
Summary
Two serine proteinase inhibitors from seeds of the bitter gourd, BGIA (bitter gourd inhibitor against acidic amino acid-specific proteinase of Streptomyces griseus) and BGIT (bitter gourd trypsin inhibitor), were crystallized for X-ray structure determination. Crystals of BGIA belong to the monoclinic space group C2 with cell dimensions of a = 54.0 Å , b = 23.7 Å, c = 47.9 Å and, β= 105.4° , and diffracted X-ray up to 1.5 Å resolution. Crystals of BGIT belong to the triclinic space group Pl with cell dimensions of a = 22.8 Å , b = 23.5 Å, c = 28.4 Å, α = 93.1 °, β = 99.6 °, and γ = 101.0 ° , giving X-ray diffraction of over 1.2 Å resolution. Intensity data of BGIA and BGIT crystals were collected using synchrotron radiation up to 1.7 and 1.4 Å , respectively.
Key words: crystallization; proteinase inhibitor.
TARAに関する表現
Acknowledgements; A.N. and I.T.are members of the TARA project.
12. 月原冨武(阪大)、山口宏(関西学院大)
Redox-Coupled Crystal Structural Changes in Bovine Heart Cytochrome c Oxidase
Science 280, 1723-1729 (1998)
Shinya Yoshikawa1, Kyoko Shinzawa-Itoh1. Ryosnke Nakashima1, Rieko Yaonon1, Eiki Yamashita2,, Noriko Inoue2, Min Yao2, Ming Jie Fei2, Clare Peters Libeu1, Tsunehiro Mizushima2, Hiroshi Yamaguchi3. Takashi Tomizaki2, Tomitake Tsukihara2
1Departnlent of Life Science. Himeji Institute of Technology and CREST, Japan Science and Technology Corporation, Kamigori Akoh. Hyogo 678-1297, Japan.
2The Institute for Protein Research. Osaka University, 3-2 Yamada-oka, Suita 565-0871. Japan
3The Faculty of Science. Kwansei Gakuin University, Uegahara. Nishinomiya Hyogo 662, Japan.
Summary
Crystal structures of bovine heart cytochrome c oxidase in the fully oxidized, fully reduced, azide-bound, and carbon monoxide-bound states were determined at 2.30, 2.35, 2.9, and 2.8 angstrom resolution, respectively. An aspartate residue apart from the O2 reduction site exchanges its effective accessibility to the matrix aqueous phase for one to the cyiosolic phase concomitantly with a significant decrease in the pK of its carboxyl group, on reduction of the metal sites. The movement indicates the aspartate as the proton pumping site. A tyrosine acidified by a covalently linked imidazole nitrogen is a possible proton donor for the O, reduction by the enzyme.
TARAに関する表現
T.Tsukihara and S.Y. are members of the TARA project of Tsnkuba University.
13. 神谷信夫(理研)
Crystallization and preliminary X-ray diffraction studies of bleomycin-binding protein fiom bleomycin-producing Streptomyces verticillus
Acta Cryst.. D54. 127-128 (1998)
Takanori Kumagai1, Kengo Muta1, Yasuyuki Matoba1, Yoshiaki Kawano2, Nobuo Kamiya2, Julian Davies3. and Masanori Sugiyama1
1Institute of Pharmaceutical Sciences, Hiroshima University School of Medicine, Kaswni 1-2-3, Minami-ku. Hiroshima 734, Japan.
22The Institute of Physical and Chemical Research (RJKEN), Spring-8. Kamigori, Hyogo 678-12, Japan.
3'Departrnent of Microbiology, and Immunology, The University of British Columbia, 300-6174, University Boulevard, Vancouver, BC, V6T IZ3, Canada.
Summary
A bleomycin-binding protein (BLMA) produced by bleomycinproducing Streptomyces verticullus was crystallized in a form suitable for X-ray diffraction analysis using the vapor-diffusion method. Crystals were grown at pH 5.7. in 0.2 M NH4 acetate and 0.1 M Na acetate. using 30% PEG 4000 as a precipitant. They belong to the orthorhombic system. with space group F21212, cell dimensions a = 54.90. b = 67.94. c = 35.60 A . and one BLMA molecule in the asymmetric unit. The crystals diffract X-rays well and the diffraction intensity data was collected up to I .5 A resolution with a merging R value of 0.054 at beamline 6B of the Photon Factory. The diffraction data set is 94% complete.
TARAに関する表現
所属に記載; Foot note; 'TARA(Tsukuba Advanced Research Alliance) guest researcher for the Sakabe project), 及び
Acknowledgements; This research was canied out using BL-6B constructed by the TARA Sakabe project. We are grateful to have had the opportunity to use the TARA Beamline.
14.神谷信夫(理研)
Novel non-heme iron center of nitrile hydratase with a claw setting of oxygen atoms
Nature Structural Biology 5, 5, 347-351
Shigehiro Nagashima1,2, Masayoshi Nakasako3, Naoshi Dohmae1. Masanari Tsujimura1,4 Koji Takio1, Masafumi Odaka1, Masaftimi Yohda1, Nobuo Kamiya1,5 and Isao Endo1
1The Institute of Physical and Chemical Research (RJKEN). Hirosawa 2-1. Wako, Saitama 351-0198, Japan.
2Present address: Namba Photonic Nanomachine Project, ERATO. JST, Hikaridai 3-4, Seika-cho, Soraku-gun, Kyoto 619-0237, Japan.
3Presto. JST and Institute for Molecular and Cellular Biosciences, The University of Tokyo. Yayoi l-1-1. Bunkyo-ku. Tokyo 113-0032, Japan.
4Gruduate School of Science and Engineering, Saitama University. Shimo-Okubo 251, Urawa. Saitama 338-0825, Japan.
5TARA Sakabe Project. University of Tsukuba. Tennodai 1-1-1, Tsukuba-city. Ibaraki 305-0006, Japan.
Summary
The iron-containing nitrile hydratase (NHase) is a photoreactive enzyme that is inactivated in the dark because of persistent association with NO and activated by photo-dissociation of NO. The crystal structure at I .7 Å resolution and mass spectrometry revealed the structure of the non-heme iron catalyic center in the nitrosylated state. Two Cys residues coordinated to the iron were post-translationally modified to Cys-sulfenic and -sulfinic acids. Together with another oxygen atom of the Ser ligand, these modifications induced a claw setting of oxygen atoms capturing an NO molecule. This unprecedented structwe is likely to enable the photo-regulation of NHase and will provide an excellent model for designing photo-controllable chelate complexes and, ultimately, proteins.
TARAに関する表現
所属に記載および
Acknowledgements; The beam time at the TARA (Tsnkuba Advanced Reseaqrch Alliance) beamline, BL6B, was given to N.K. as a member of the TARA project.